
I’m sure you’ve all had the experience of encountering something mysterious or unknown while out and about, thinking to yourself something along the lines of, “Hmm, I should really look into this further when I get home,” but then getting distracted, forgetting to do so, and as a result, finding yourself in the exact same situation over and over again. This happens to me all the time.
For me, the absolute paragon of this experience plays out every time I’ve ever filled up the gas tank in my car. It plays out the same way each and every time: I pull up, unscrew the gas cap, slide my credit card into the machine, enter my zip code, and then, automatically and unthinkingly, press the leftmost of the array of buttons presented to me, which usually has the number 87 written on it. As I wait for the tank to fill, I’m granted a 1-3 minute-long space of idle contemplation, which I inevitably fill with the following thoughts:
Why are there multiple choices of gasoline? What’s the difference between them? What do these numbers even mean? Is it worth it to get the more expensive option that has a higher number? Is it somehow better for your car? And wait, if it is, then who on earth is buying the middle option? I really should look into this further when I get home…
But then the pump makes that clink sound and all thoughts of the matter are banished from my head until the next time I pull up to a tank. Until now, that is.
While looking into this, I quickly realized that I was missing quite a few of the fundamentals of how a car engine works. I knew that there was some exploding happening in there, and I knew that there were things in there called fuel injectors, pistons, as well as something called a ‘spark plug,’ but I wasn’t really too clear on how that all fit together.
As it turns out, this is all very important for understanding why there are multiple types of fuel, so let’s begin with a brief review of the reason we produce and purchase gasoline for our cars in the first place: the internal combustion engine.
The Four-Stroke Internal Combustion Engine
Developed throughout the mid- to late-19th century and brought to wide commercial use in the 20th, the internal combustion engine was and is one of the most important technologies shaping our modern world. (But here’s hoping that it becomes decreasingly so as electric motor technologies continue to develop.)
Before internal combustion engines, the most powerful type of engine was the steam engine, which can be said to be an external combustion engine because the burning of fuel was done outside of the engine (think of the dedicated coal burning cars on old steam-powered train engines). The critical innovation of the internal combustion engine is right there in the name: the combustion of the fuel is done inside the engine, allowing for smaller sizes and increased efficiency.
Although there are many types of internal combustion engines (gasoline, diesel, turbine, to name a few), they all set out to accomplish the same thing: convert chemical potential energy (in the form of a fuel source) into mechanical energy (the turning of wheels) through combustion (by exploding it!). For the purposes of this article, I’m going to talk about the most common type of internal combustion engine, the one you probably have in your car: the gasoline four-stroke engine.
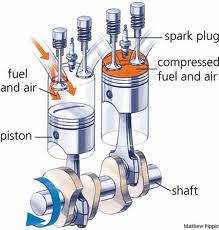
First, a brief overview of the important components of the four-stroke engine: the piston, the chamber, the spark plug, and the crankshaft. The piston is a metal cylinder that moves up and down inside of a cylindrical chamber. This chamber is where a mixture of fuel and air will be pumped and eventually combusted. The spark plug, which we’ll get to in a minute, is located at the top of the chamber. The piston, which moves up and down in the chamber, is attached by a rod to the crankshaft, which converts this up-and-down mechanical motion into rotational motion, causing the wheels of the car to turn.
The four-stroke engine is so called because a complete cycle involves four movements, or “strokes,” of the piston. Each one of these strokes can be said to represent a different phase of the cycle. I’m including a brief description of each phase, but we’re mostly going to focus on phases 2 (Compression) and 3 (Combustion) for the rest of this article.
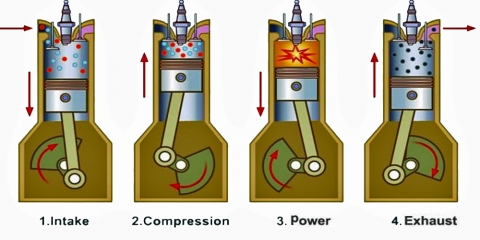
- Intake — the phase in which the piston moves down, increasing the total volume of the chamber and sucking in a mixture of fuel and air
- Compression — the phase in which the piston moves back up, compressing the fuel-air mixture in anticipation of ignition
- Combustion — also known as the “power” phase, because the compressed fuel-air mixture is ignited and detonates, the power of which forces the piston down and sets the whole cycle in motion
- Exhaust — the phase in which the piston moves back up, expelling the expended fuel-air mixture as exhaust
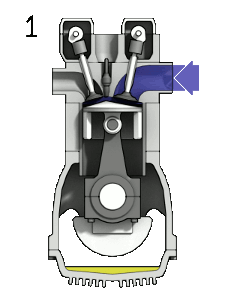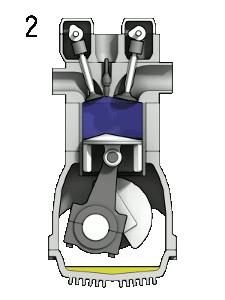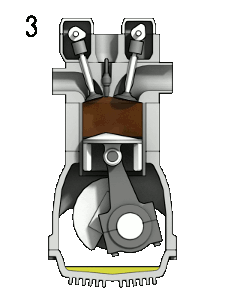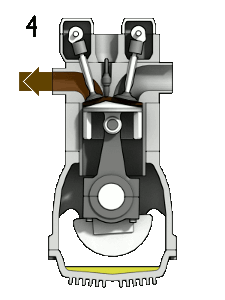
This whole cycle happens really, really fast. (A full revolution of the crankshaft occurs every two strokes, so if your engine is running at 2000 rpm, that means this entire cycle is happening 1000 times each minute.)
Now, in order to finally get at what the difference between different types of gasoline are, let’s zoom in on the compression and combustion phases of this cycle. In order to do so, we’ll have to understand a concept called the “compression ratio” of an engine. The compression ratio is the ratio of the volume of the chamber between when the piston is all the way at the bottom to the volume of the chamber when the piston is all the way at the top. In other words, the number represents by how much the fuel-air mixture in the engine gets compressed during the compression phase. In many car engines, this ratio is something like 8:1, but in some high-performance vehicles, this ratio can be higher, like 12:1.
Why this difference in engine compression ratios? Well, for a variety of reasons, higher compression ratios result in increased power, higher fuel economy, and lower emissions. So what’s the catch? Why doesn’t every engine have a super high compression ratio? The answer to this question is the core of why premium gasoline exists. Essentially, it involves heat.
Compression generates heat. The more a gas is compressed, the hotter it gets. (Remember the ideal gas law?)
In a properly functioning gasoline engine (diesel is a slightly different story, the fuel-air mixture should not detonate until the combustion phase of the cycle. Remember the spark plug? In the combustion phase the spark plug provides, well, a “spark” that ignites and detonates the compressed mixture, forcing the piston down and powering the cycle.
The reason this is desirable has to do with the spread of the flame throughout the mixture. In order to most efficiently convert the energy stored in the fuel to mechanical energy—in other words, to most efficiently push the piston down—a smooth, consistent explosion is required, one that begins in the top middle (where the spark plug is) and evenly spreads down and throughout the chamber.
If the fuel-air mixture is compressed too much and becomes too hot, however, detonation events can occur in other parts of the chamber before the spark plug fires, which causes all kinds of problems. This self-detonation phenomenon is often called “knocking” or “pinging” due to the audible sound that it can cause.
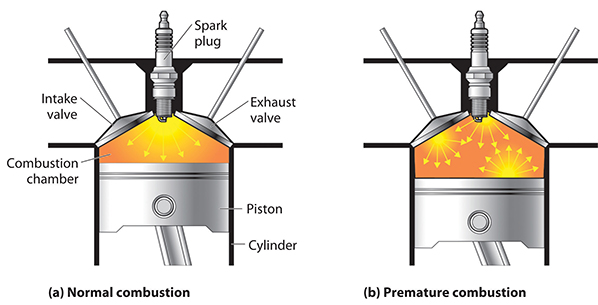
So, how can you use a higher compression ratio (and thus make the fuel to movement conversion more efficient) without running the risk of heating up the chamber to the point where the fuel begins to spontaneously combust on its own? The answer: higher octane fuels, otherwise known by their marketing name “premium gasoline.”
High Octane Gasoline
Types of gasoline are differentiated by their octane ratings—as a general rule, the higher the octane rating, the more “premium” (meaning expensive) it is. Octane ratings are the numbers that you see on the different gasoline options at the gas pump. The kind that you probably put into your car has an octane rating of 87, whereas premium fuel can have a rating of 91 or 93.
What do these numbers mean and where do they come from? To put it summarily: the higher the octane rating of a type of gasoline, the more it can be compressed and heated before self-detonating. If you were to use a lower octane fuel in a high-compression engine, the increased temperatures from compression might cause the fuel to prematurely detonate, causing the “knocking” problem mentioned above. This problem could be obviated by supplying the engine with a higher-octane fuel, which is able to better withstand the increased temperature of the high compression engine and will not detonate until the proper moment in the engine’s cycle, when the spark plug ignites it.
If you are like me, you might at first assume that the octane rating measures the amount of octane that is in a particular type of gasoline. As it turns out, this is only partially correct. Most modern gasoline types are actually a complex mixture of different elements (of which iso-octane is indeed a key component). In order to arrive at a standard system of measurement for all of these different mixtures, however, each fuel is tested against a reference mixture of pure iso-octane (octane rating of 100) and n-heptane (octane rating of 0) to determine its actual octane rating.
A fuel mixture that begins to self-ignite under the same conditions as a mixture of 87% iso-octane and 13% n-heptane is said to have an octane rating of 87, even though the fuel mixture itself might be composed of many different chemicals and substances. And this is important, because one way of increasing a fuel’s efficiency and performance is to increase its octane rating, which is done through the use of a variety of chemical compounds. Until lead-infused gasoline was banned in the United States in the early ‘90s, lead was a popular additive for exactly this reason. Today, most the motor fuel consumed in the United States is composed of at least 10% ethanol, a high-octane fuel which, when blended with otherwise “pure” gasoline, increases its octane rating and allows for higher performance (as well as lower carbon emissions). Even though Ethanol has a lower energy output than other components of gasoline, the increased octane rating of fuel mixtures containing ethanol allows modern engines to run at higher compression ratios, enabling engines to preserve or even increase their total fuel efficiency.
There are two major standardized methods for testing gasoline and measuring its octane rating: the Research Octane Number (RON) and the Motor Octane Number (MON). The differences between the two have to do with the conditions under which the fuels are tested, but if you live in the United States, these differences needn’t worry you because the most common reported rating is actually an average of the two. Next time you are at a gas pump and look at the octane ratings, you’ll probably notice the following text: (R+M)/2.
Conclusion
So, next time you are at the gas pump, which variety should you use? Well, unless you are driving a vehicle whose highly-efficient, high-performance engine requires higher octane fuels, it’s unlikely that you are going to get any benefit from purchasing the more expensive, higher-octane fuel. (Although maybe there is something to be said for the placebo peace-of-mind that some buyers might experience, treating their car to “a special treat” every once in a while?) You might as well save yourself the money and just go with the lower-octane stuff. Unless you have a problem with engine knocking, your car probably doesn’t need high-octane fuel.
But if you do happen to own a car that is supposed to take premium fuel, are you going to harm it if you buy the cheap stuff? The consensus seems to be that modern engine monitoring systems are good enough to be able to adjust the timing of the ignition in order to account for the use of lower octane fuels, meaning that it’s unlikely that you will do damage to your engine by causing knocking. But by not utilizing the maximum efficiency of your car’s engine, you might not actually be saving yourself much money and you may be emitting more carbon dioxide than you need to. Hey, you went through the trouble and money to buy one of these fancy cars, I’m sure you can afford to pay a little more at the pump and let those other buttons do their job.